Written by Amna Khan-Patel Chief Pharmaceutical Officer’s Clinical Fellow 23/24
Across the NHS in England, over 237 million medication errors annually are reported.1 The BMJ Quality and Safety study reveals errors at all stages of the medicine process, but highlights 54.4% occurring during administration.2 These errors result in patient harm, prolonged hospital stays, and increased healthcare expenses. Closed loop medicine administration (CLMA) offers a digital remedy to mitigate these errors and enhance patient care.
What is CLMA?
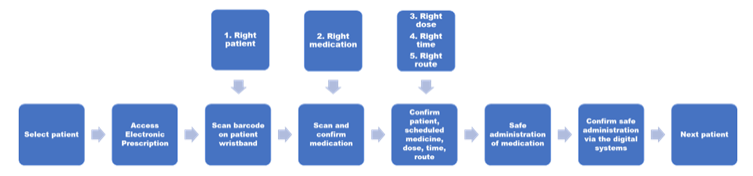
CLMA uses digital barcode technology to provide additional validation when administrating medication. Barcode scanning identifies patients correctly to receive correct medications, in accordance with the 5 rights. The process of CLMA is (diagram 1):
1. During medication rounds the user (nurse) accesses the patient’s electronic prescription and scans the barcode on the patient’s wristband. This confirms the patient’s identity and automatically identifies any medications due to be administered.2. The user scans the barcode on the medication box to confirm the 5 rights – right patient, right medication, right form, right route and right dose.
3. If all of these are correct the medication will confirm a ‘match’ and the user can prepare the medication ready for administration.
4. If any of the 5 rights are incorrect, for example an incorrect medication is scanned, then a warning message appears on the prescription. This prevents administration and prompts the user to check the medication, dose etc. This stage in the process acts as a second check.
5. Once all medications have been correctly scanned these can be safely administered to the patient.
Diagram of CLMA
Global Digital Exemplar (GDE) Sites and Scan4Safety (S4S)
In 2019 GDE sites were supported to implement CLMA as part of the accelerated uptake of Electronic Prescribing and Medication Administration (EPMA) systems. The early adopters sites made significant progress with CLMA and began to address the technical, and procedural issues. The findings from this programme of work have been shared in the Academic Health Science Network (AHSN) Closed Loop Medicine Administration Toolkit – Implementation Lessons Shared.
The Scan4Safety initiative aims to improve patient safety through accurate point-of-care scanning and streamlining data entry processes and documentation. CLMA is not an essential requirement in the S4S programme, however, there is clear alignment with the objectives of the S4S and those for CLMA.Scan4Saftey uses Global Standards One (GS1) for product, location and patient identification. The systems tracks products from the point of manufacture to the point of care.
NHS Information Standards
NHS England’s Digital Clinical Safety Strategy promotes Scan4Safety to enhance patient safety, traceability, operational productivity as well as supply chain efficiency. Recommended standards for implementing CLMA include:
SCCI0052: Dictionary of medicines and devices (dm+d)
A dictionary of UK licensed medicines that supports interoperability - Electronic systems exchanging or sharing patient-related medication information must adhere to the standard by using dm+d identifiers and descriptions for data exchange. This standard currently excludes medical devices.
DAPB0108: Automatic Identification and Data Capture (AIDC)
Applies to any NHS setting in England where AIDC technologies are used as enablers to improve patient safety, ensure greater clinical effectiveness and drive operational efficiencies
DCB1077: AIDC for Patient Identification
The AIDC for patient identification outlines encoding NHS approved patient identifiers into GS1 DataMatrix barcodes (a two dimensional barcode), covering production, verification, and printing rules. Implementing these barcodes supports accurate, timely, and safer patient identification in England.
Benefits of CLMA
CLMA provides the opportunity to reduce medication administration errors whilst identifying the types of errors that have been avoided. It collects data on these avoided errors, users, and frequency, facilitating trend analysis, process reviews, and scanning compliance rates. Adoption of CLMA advances digital maturity, a key aspect of HIMSS EMRAM accreditation. Trusts that have implemented CLMA report improved patient safety by reducing medication errors through barcode scanning, which alerts to prevent potential errors.
Opportunities Ahead
With all innovative technology there are unforeseen challenges. NHS Trusts adopting CLMA face issues including medications without barcodes, barcodes placed on packaging rather than bottles and unlisted patient’s own medications to name a few. Potential solutions include manufacturers labelling the product directly and organisations reviewing their work flows.
Despite not yet being widely adopted across England, CLMA holds promise for improving patient safety. Where CLMA is implemented NHS trusts have focussed on its use in administering solid oral dose medication, the potential use of CLMA for intravenous medication has not been established. The NHS Digital Medicines programme is reviewing CLMA’s current landscape to understand the complexities, benefits and challenges for adopting CLMA, including intravenous single and multi-dose medications. As part of NHS England’s Digital Medicines First of Type scheme Liverpool Heart and Chest Hospital project aims to develop solutions that support efficiencies and safety improvements relating to CLMA or supply focusing on the adoption of CLMA for intravenous medication, these findings will be shared with the wider NHS to enable to allow others to fast follow.
Fellowship Project
 I am Amna Khan-Patel Chief Pharmaceutical Officer’s Clinical Fellow 23/24 working at NHS England Transformation Directorate within the Digital Clinical Informatics team. I am a secondary care pharmacist and have completed my MSc in Secondary Care, which focused on the implementation of the electronic pharmaceutical care plan to improve patient care.
I am Amna Khan-Patel Chief Pharmaceutical Officer’s Clinical Fellow 23/24 working at NHS England Transformation Directorate within the Digital Clinical Informatics team. I am a secondary care pharmacist and have completed my MSc in Secondary Care, which focused on the implementation of the electronic pharmaceutical care plan to improve patient care.
As part of the fellowship year, I am leading on the CLMA project to understand the current situation of CLMA across England and what the future opportunities are. The project focuses on identifying the benefits, challenges and obstacles by revisiting the original GDE sites that were commissioned to implement CLMA. Working with GS1 to understand if there are common challenges or barriers for adoption how they can be overcome. If your organisation has implemented or is developing work processes for the adoption of CLMA and are interested in being involved in the project to share learning please contact me: amna.khan-patel@nhs.net.
HETT North 26th February – Healthcare Excellence Through Technology
Find out more about CLMA at HETT North on 26th February at Manchester Central.
References
- Elliott RA, Camacho E, Jankovic D, et al Economic analysis of the prevalence and clinical and economic burden of medication error in England BMJ Quality & Safety 2021;30:96-105. https://doi.org/10.1136/bmjqs-2019-010206
2. Truitt, E. Effect of the Implementation of Barcode Technology and an Electronic Medication Administration Record on Adverse Drug Events. Hosp Pharm. 2016;51(6): 474-483.
%20(1).png?width=500&height=58&name=HETT%20insights%20logo%20RGB-04%20(1)%20(1).png)


.png)
